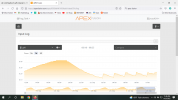
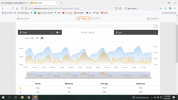
It's starting to happen again, my high pH alarm is starting to go off just like it did towards the end of last winter. For some reason when the weather gets cooler, my pH climbs to 8.5+ during the light cycle. Typically the alarm will go off an hour or so before my lights kick off. Why is this happening? I haven't changed any inputs and it really does seem to be caused by cooler temps outside. The alarm went off a few weeks ago when we had 2-3 cooler nights and, after last night, the pH started quite a bit higher than it usually does. If you look at the past pH, you can see that if the day was a hot one, the pH would be lower. September 18th & 19th still had cooler night but the last few days have been really warm. I have no idea why cold weather would alter the pH so much. Has anyone experienced this?
A few things to note: 1. The tank is in a basement with lots and lots of ventilation and no conditioned air. In Winter the air temp can get be in the low 60's if it's really cold outside. 2. It has been 9 months since I calibrated my pH probe. I don't believe this is the root cause, however, because I experienced the same high pH last winter after correctly calibrating the pH probe. 3. It's a fairly large space and there's never more the one person down there (Me) and even then it's not for very long. i.e. there aren't any folks exhaling and putting CO2 into the air.
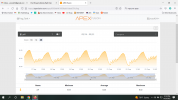
A few things to note: 1. The tank is in a basement with lots and lots of ventilation and no conditioned air. In Winter the air temp can get be in the low 60's if it's really cold outside. 2. It has been 9 months since I calibrated my pH probe. I don't believe this is the root cause, however, because I experienced the same high pH last winter after correctly calibrating the pH probe. 3. It's a fairly large space and there's never more the one person down there (Me) and even then it's not for very long. i.e. there aren't any folks exhaling and putting CO2 into the air.